With our partners at German Spine Society (DWG), we’re actively deploying an innovative data solution at clinical institutions in Germany, Austria and Switzerland to ensure the quality of spine surgery treatments. Learn how we’re scaling healthtech to improve patient treatments for today and tomorrow.
Challenge
Quality control is a growing focus in healthcare with the increased need for evidence-based studies advancing the effectiveness of therapies. DWG recognizes that outcomes research is a direct driver and benchmark for standard of care. The opportunity lies in improving service quality, resource efficiency and above all patient health—delivering both clinical and economic value.1
Solution
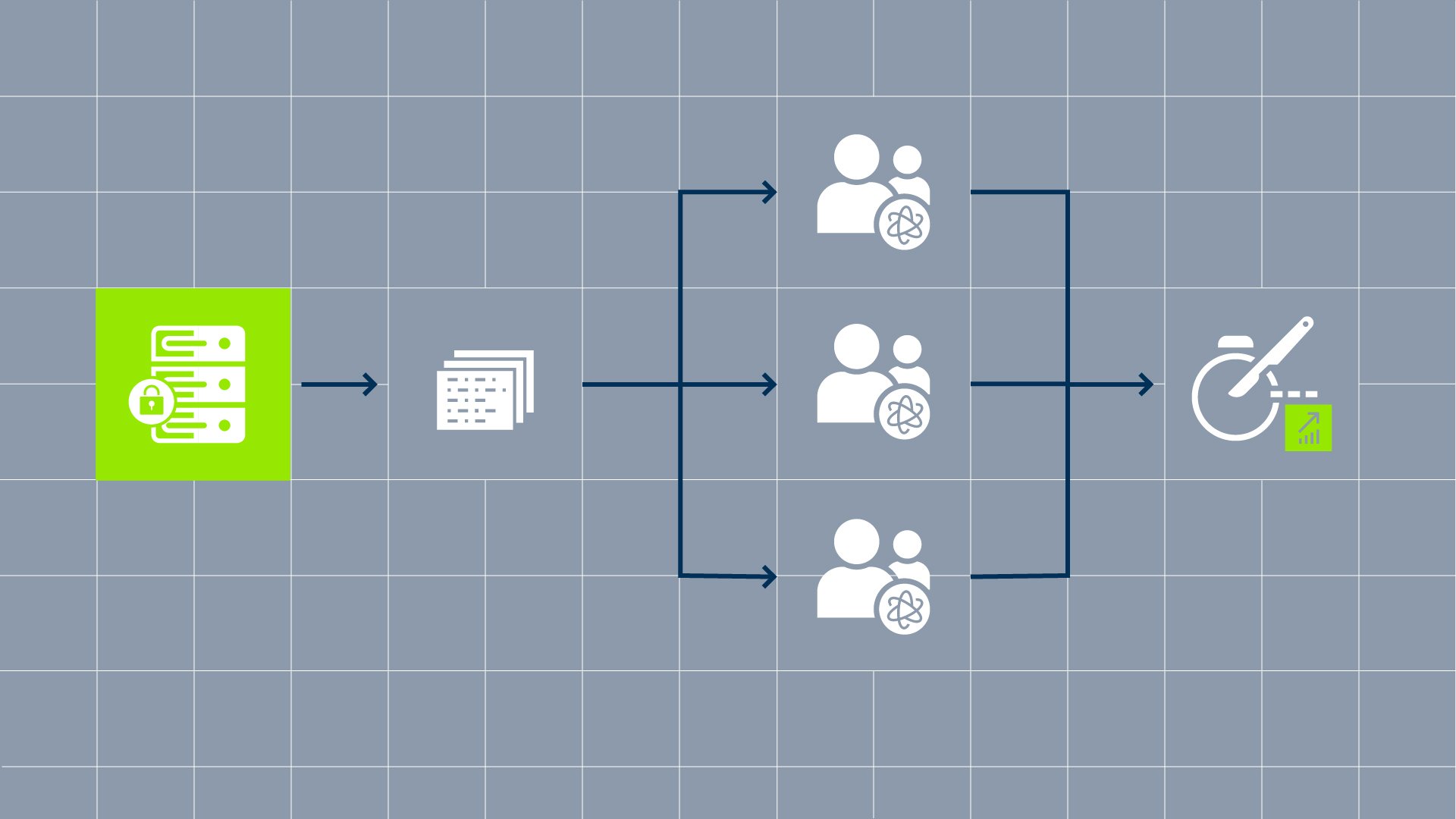
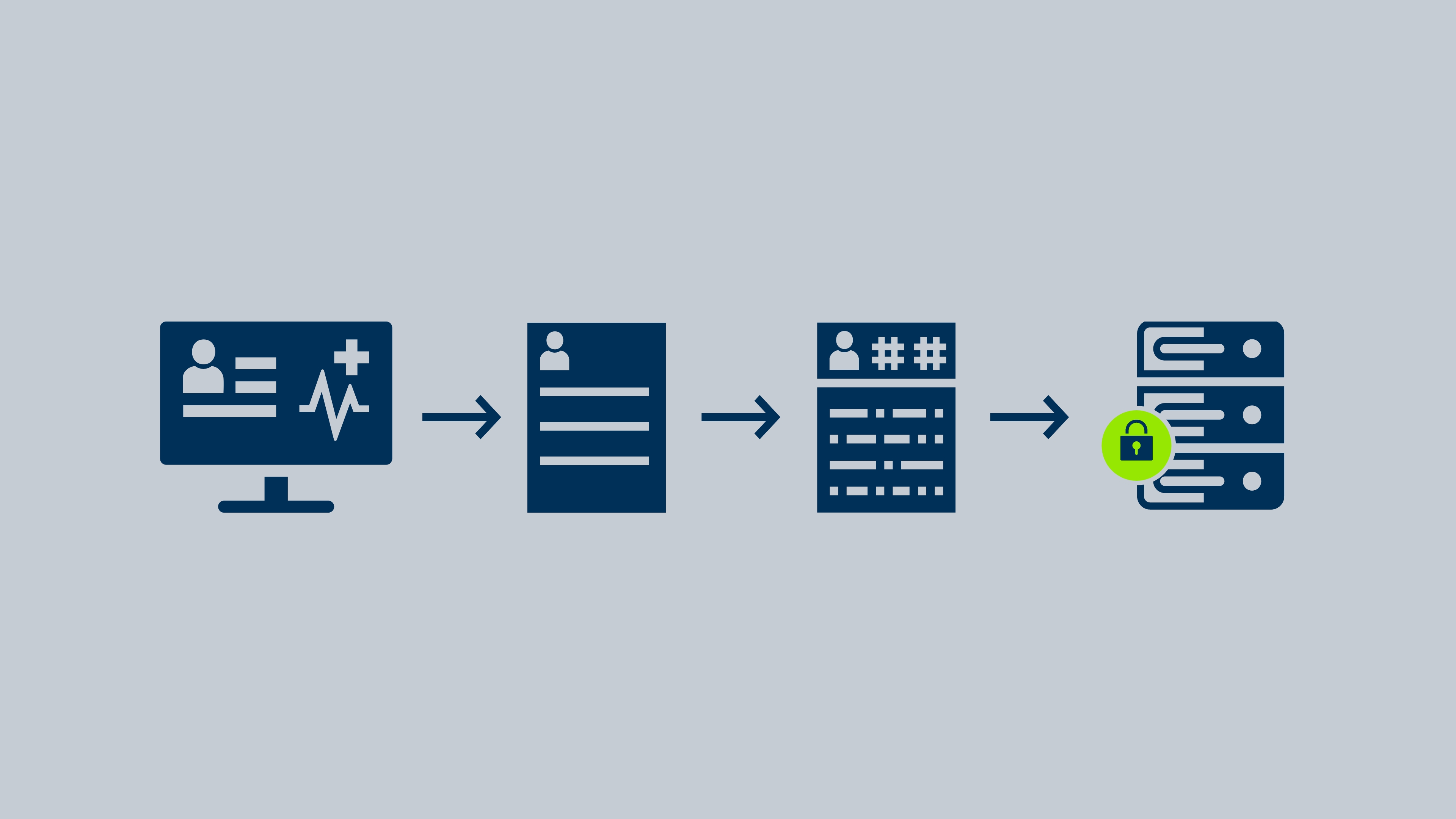
DWG and Snke collaborated to create an innovative data solution that integrates a secure, cloud-based repository with tools for structured data acquisition, de-identification of patient records, a robust patient consent framework, and an independent pseudonymization system. This comprehensive approach enables the secure collection, processing, and analysis of clinically relevant data while maintaining patient privacy at every stage of the data lifecycle.
Challenge
Quality control is a growing focus in healthcare with the increased need for evidence-based studies advancing the effectiveness of therapies. DWG recognizes that outcomes research is a direct driver and benchmark for standard of care. The opportunity lies in improving service quality, resource efficiency and above all patient health—delivering both clinical and economic value.1
Solution
DWG and Snke collaborated to create an innovative data solution that integrates a secure, cloud-based repository with tools for structured data acquisition, de-identification of patient records, a robust patient consent framework, and an independent pseudonymization system. This comprehensive approach enables the secure collection, processing, and analysis of clinically relevant data while maintaining patient privacy at every stage of the data lifecycle.
Put Collaboration Dataspace
to work
Integrate to elevate the standard of care.
Scaling Snke with the German Spine Registry
Empowering research
Participating clinics upload relevant data to the registry solution via our reporting software. In the DWG registry study, the focus is on the patients at all times: Data collection begins with patient consent via our Data Sharing Consent framework. To protect the patients’ identities, the data is pseudonymized prior to upload with the help of an external trust center. This means that patients cannot be identified when the data is used.
Take the next step
Unlock the power of the German Spine Registry for your clinic.